News
2025-06-06 15:45:06 Views:412
The Perlove Medical integrated C-arm with large FPD Receives FDA 510(k) Clearance

We are pleased to announce that Perlove Medical integrated C-arm with large FPD, the PLX119C Series, has officially received FDA 510(k) clearance from the United States Food and Drug Administration (FDA). This approval marks a significant milestone, allowing the product to be legally marketed in the U.S. and further accelerating Perlove Medical’s global expansion.

About FDA 510(k)
The U.S. FDA (Food and Drug Administration) is one of the most stringent regulatory bodies in the world, overseeing industries including food, pharmaceuticals, medical devices, and cosmetics. FDA certification ensures that products sold in the U.S. meet strict standards for safety, effectiveness, and quality, safeguarding public health. Products approved by the FDA are recognized globally for their reliability and safety.
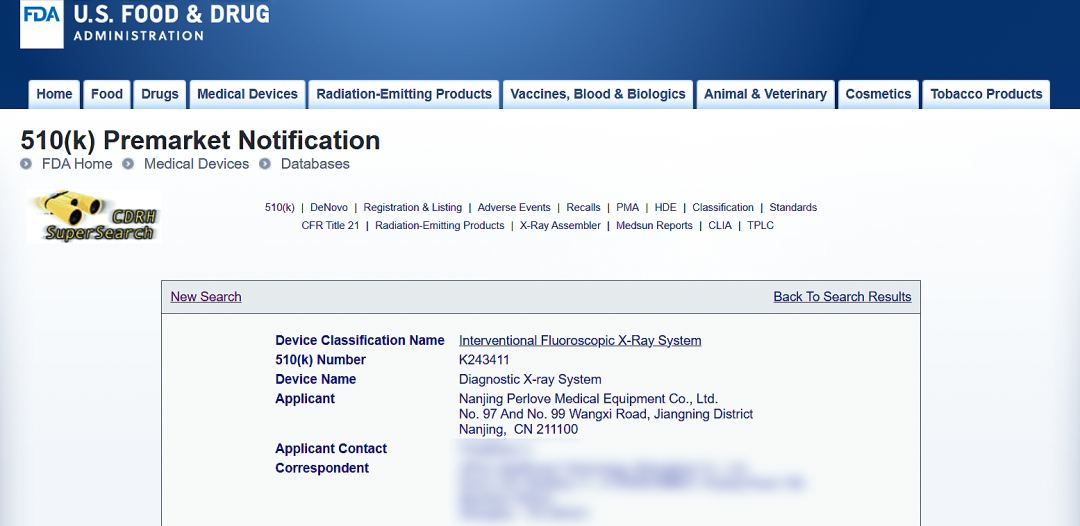
The recent FDA 510(k) clearance of Perlove Medical integrated C-arm with large FPD follows the earlier approval of our high-power mobile DR series. These milestones demonstrate our continued commitment to international compliance and quality, especially amid growing global competition in the medical device industry. Perlove Medical is rapidly advancing its international strategy, marking a new chapter in our global journey.


High product quality is the key to entering international markets. At Perlove Medical, we strictly adhere to international standards throughout the entire manufacturing process—from raw material procurement and component fabrication to final assembly. Every step is executed with precision to ensure the stability and reliability of our products.
Designed with a wide field of view and highly integrated structure, the PLX119C large flat panel C-arm meets the diverse needs of operating rooms around the world. It delivers comprehensive imaging and intuitive operation, the equipment is able to provide accurate image support for both intricate orthopedic procedures and complex surgeries in other specialties.
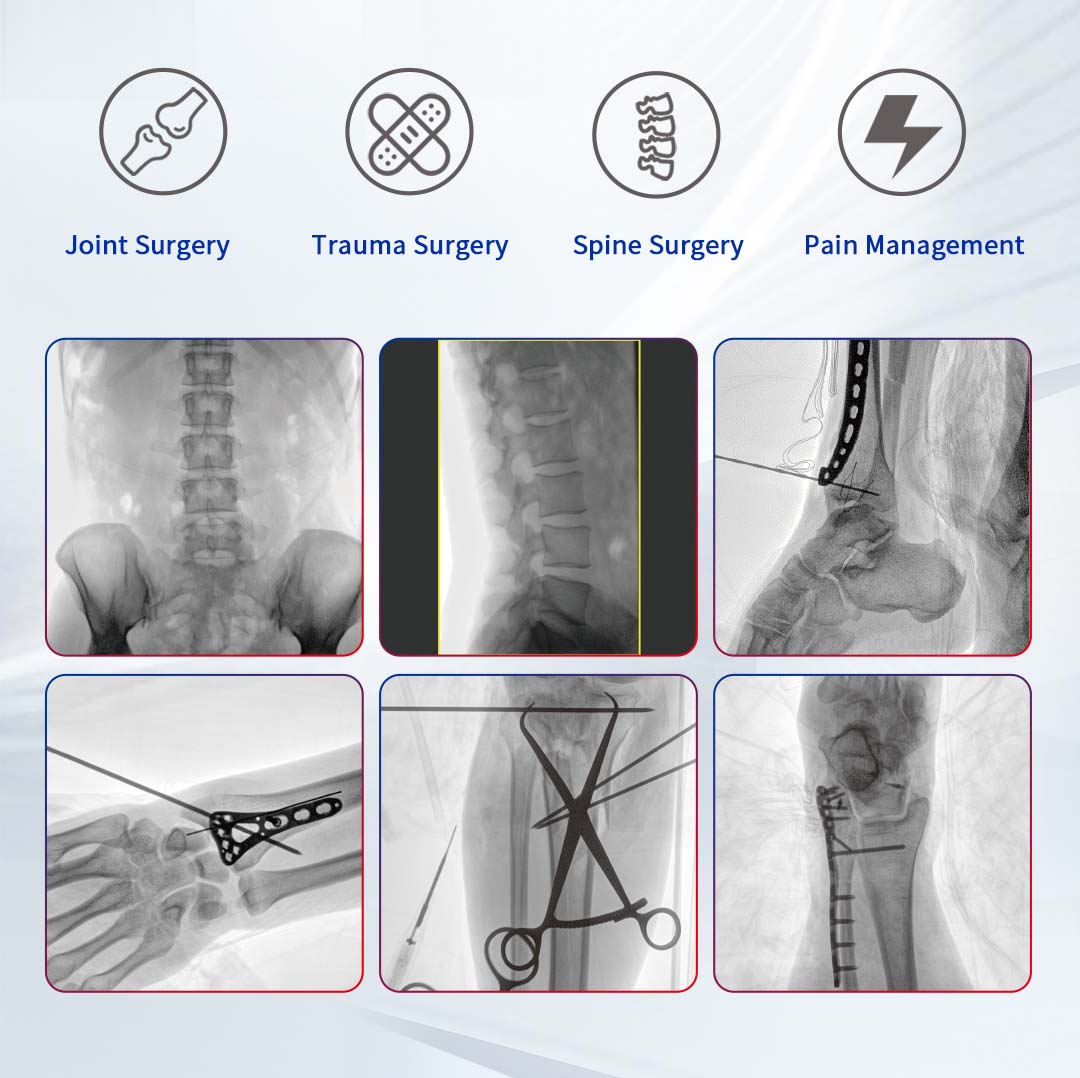
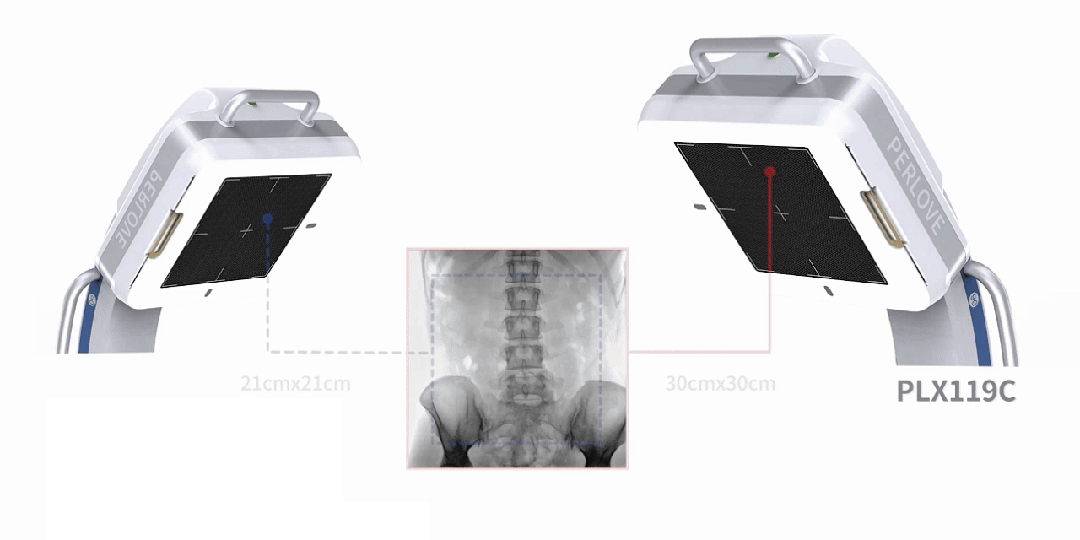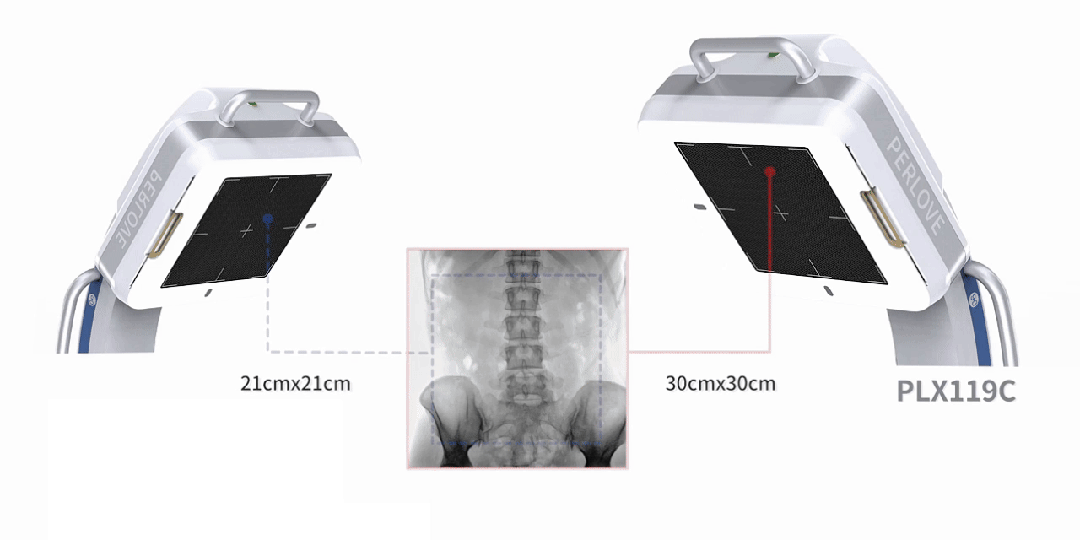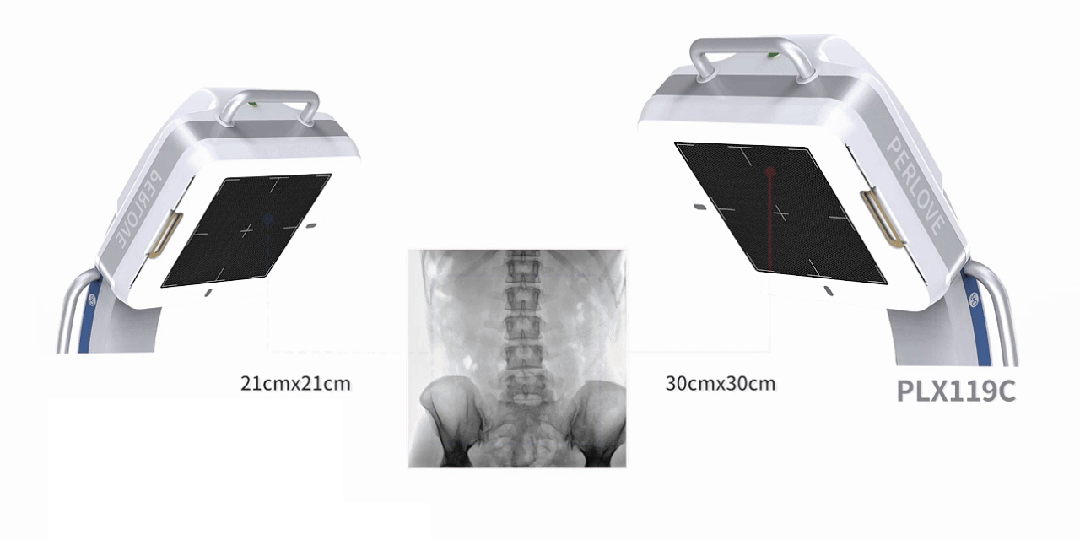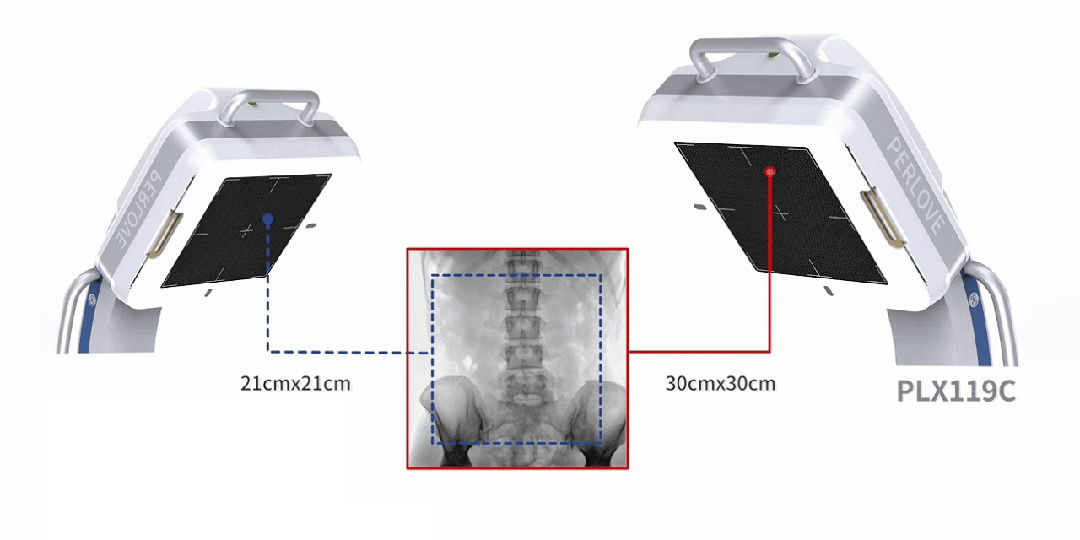
Enhanced Imaging, Doubled Efficiency
Equipped with a 30cm × 30cm flat panel detector, the PLX119C provides a broader field of view in a single image acquisition. Surgeons can observe the lesion and surrounding tissues in detail at once, significantly improving surgical accuracy and efficiency.
 All-in-One Design, Flexibility and Convenience
All-in-One Design, Flexibility and Convenience
With an integrated design that occupies just about 1㎡ of floor space, the device is well-suited for operating rooms in different countries and regions where space may be limited or layouts are complex. The compact system features a streamlined body with no bulky external cables, allowing for flexible installation without the need for a separate workstation. It adapts easily to various clinical environments, ensuring smooth surgical workflows.

From the Americas to Europe, from Asia to Africa…
Perlove Medical’s smart technologies have entered over 100 countries and regions worldwide.
This FDA approval is more than just a regulatory milestone—it’s a passport for entering the U.S. market and a significant leap forward in our global expansion strategy.
![]()
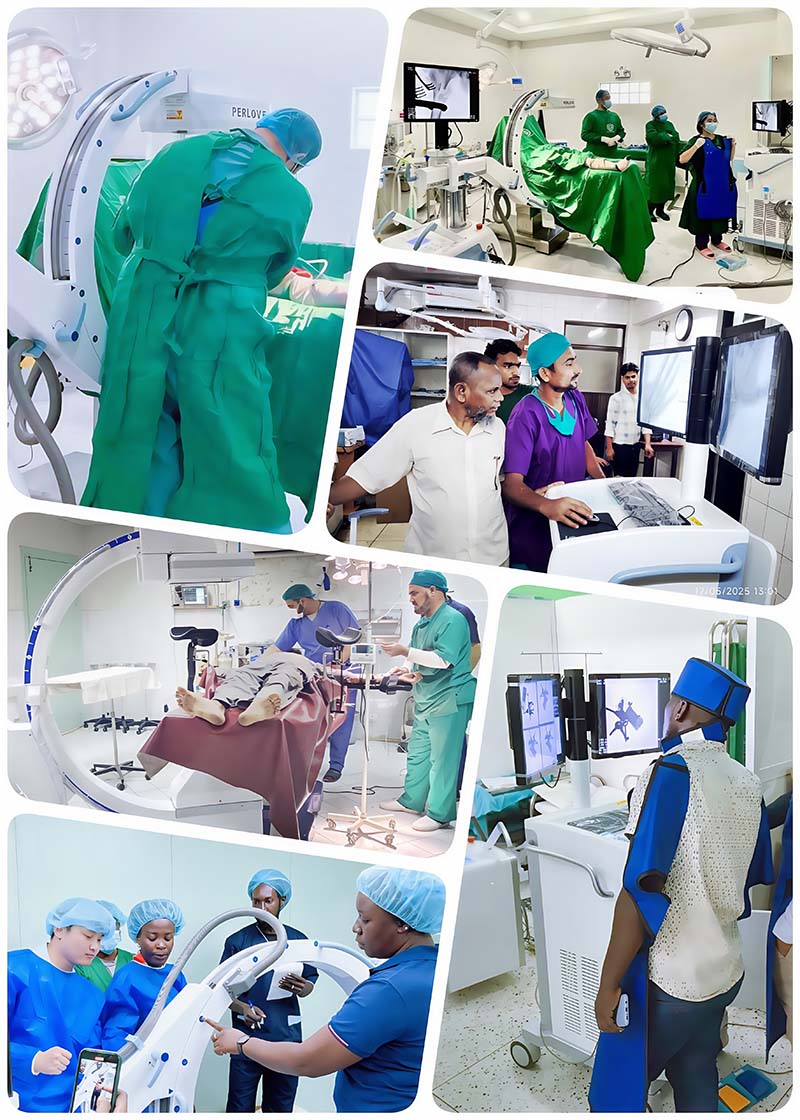
Perlove Medical will continue to closely monitor the medical market needs and regulatory differences across regions. We are committed to expanding product registration and marketing efforts worldwide, aiming to bring intelligent orthopedic surgical robots and advanced imaging systems to the global stage. Our goal is to become a globally recognized medical brand and make meaningful contributions to healthcare systems around the world.
-
Orthopedic surgical robot assists in sacral nerve stimulator implantation.
Read More » -
A Powerful Tool in Common Bile Duct Stone Removal Surgery — The Mobile Flat-Panel C-Arm For Intervention
Read More » -
Perlove Medical @CAOS 2025 | Intelligent Imaging for Precision
Read More » -
What Are the Clinical Advantages of the C-Arm Design in Medical X-Ray Systems?
Read More » -
Multilevel Vertebral Compression Fractures With Vertebral Deformities: How Can Surgical Robots Break Through the Challenge?
Read More » -
Perlove Medical Shines at FIME 2025: Explore the Future of Global Healthcare Innovation
Read More »