News
2025-10-21 18:26:20 Views:490
Another International Milestone! Multiple Perlove Medical Devices Achieve MDR CE Certification
In today’s increasingly competitive global medical device market, product compliance and quality are the core competencies for companies aiming to establish themselves internationally. The stringent standards of the European Union’s Medical Device Regulation (MDR) have become a key benchmark for evaluating product strength. Perlove Medical’s large flat-panel integrated C-arm, Split-Type mini C-arm, mobile DR series, and dynamic flat-panel DRF systems have all successfully obtained MDR CE certification. This achievement not only showcases their outstanding product quality and technological capabilities but also marks a critical breakthrough in Perlove Medical’s international journey.
MDR CE Certification
The Global “Gold Standard” for Medical Device Quality
Currently, countries around the world are imposing stricter requirements on the safety and clinical efficacy of medical devices. Among these, the EU MDR CE certification, recognized globally for its systematic and rigorous standards, has become a “quality passport.” Compared with the previous Medical Device Directive (MDD), MDR establishes a regulatory framework covering the full product lifecycle. From risk assessment in design and R&D, through quality control in manufacturing, to post-market surveillance, every step is held to strict standards. This provides dynamic recognition of a company’s continuous compliance capabilities and the core value of its products.
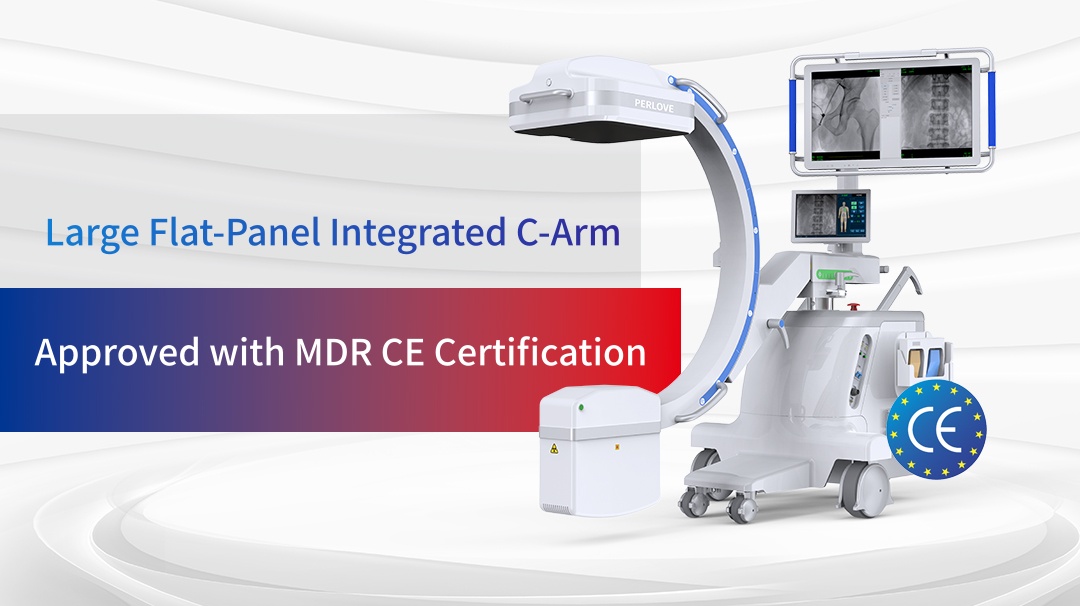



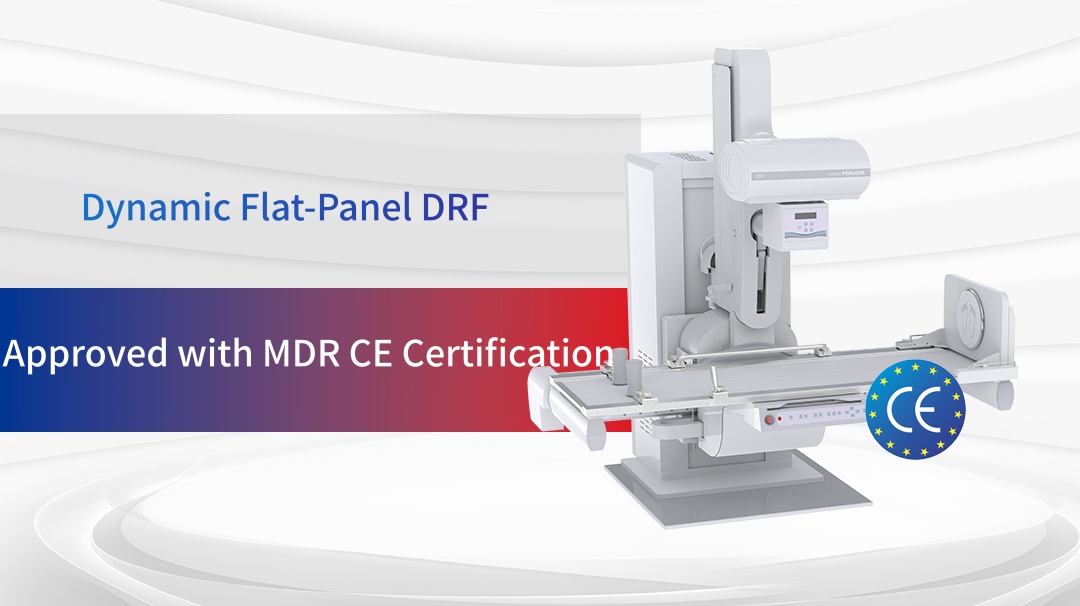
Multiple Perlove Medical Devices Obtain MDR CE Certification
Regarding clinical evidence, MDR requires companies to submit clinical trial data for their own products, and some categories even require Europe-specific data. Today, MDR CE certification has gone beyond a mere market entry label—it has become a “gold standard of quality” that reflects a company’s technical strength, quality management level, and social responsibility.
From MDD to MDR
A Fundamental Regulatory Upgrade
In May 2021, MDR officially replaced the MDD, which had been in use for nearly 30 years. This change was not just a regulatory adjustment, but a fundamental reshaping of compliance logic. Unlike the MDD, which required member states to transpose it into national law (resulting in execution differences), MDR functions as a “regulation” directly enforceable throughout the EU and European Economic Area, ensuring unified regulatory standards and consistent implementation. Products certified under MDR CE can freely circulate across the EU market, breaking regional regulatory barriers.

■ Certification Requirements Are Strengthened Across the Board
MDD and MDR differ significantly in certification detail. MDR imposes more in-depth and precise requirements across clinical evidence, risk classification, technical documentation, and post-market surveillance. Additionally, MDR introduces new demands such as software device cybersecurity assessment and full-chain traceability of the supply chain, creating a comprehensive compliance system with significantly higher certification value.
■ Expanded Corporate Responsibility
Under MDD, corporate responsibility focused largely on pre-market compliance review. MDR extends responsibility to the entire product lifecycle, requiring companies to continuously monitor the performance and safety of products after market release, proactively address adverse events, and even manage potential risks from retired products. This drives companies to continuously improve their quality management systems.
In the domestic market, as China’s second-largest supplier of medical X-ray imaging systems, Perlove Medical has leveraged 22 years of industry experience and technical expertise to solidify its local foundation. Its six major product lines—including orthopedic surgical robots, C-arms, mobile DR, fixed DR, DRF, and dental CBCT—offer hundreds of products capable of meeting “one-stop” procurement needs for orthopedic surgical robotics and general radiology across various medical institutions. Its mobile C-arm products have maintained a leading domestic market share for over a decade.
This time, multiple products achieving MDR CE certification not only demonstrate Perlove Medical’s technical strength and quality rooted in the local market but also mark a key leap toward global expansion. It signifies that our core general radiology products fully comply with EU lifecycle regulatory standards, gaining entry into the EU market and leveraging alignment between MDR and multiple international certification systems to expand into more countries and regions worldwide.

From delivering key medical projects along the Belt and Road countries such as Uzbekistan and Pakistan, to continuously developing markets in Southeast Asia and the Americas, Perlove Medical is anchoring on local technological accumulation and bridging with international high-end certifications to build a unique advantage of “strong local foundation, broad global layout” in the global medical device arena.
-
Perlove Medical Concludes a Successful Presence at RSNA 2025 in Chicago
Read More » -
Multiple C-arm Systems From Perlove Medical Installed at Zhujiang Hospital of Southern Medical University
Read More » -
Perlove Medical 3D C-arm Installed at Ningde City Hospital
Read More » -
Perlove Medical Debuts New Products at the 2025 Chinese Conference of Digestive Endoscopology
Read More » -
Perlove Medical to Showcase Innovative Imaging Solutions at RSNA 2025
Read More » -
Perlove Medical’s C-Arm in the Pain Management Department
Read More »